LKC UltraPure
Designed to meet the specific demands of the biotech, pharmaceutical and personal care industries, the LKC UltraPure non-return valve provide safe, easy installation and high, consistent quality.
UltraPure quality
LKC UltraPure is developed to facilitate safe and easy installation that ensures optimum integrity. This valve offer high and consistent quality and thorough documentation which meet the strict standards of the biotech, pharmaceutical and personal care industries.
High and consistent quality
LKC UltraPure valves are subject to rigorous quality control. Alfa Laval performs all incoming and outgoing quality control and inspections and closely monitors every aspect of the manufacturing process – from raw material sourcing to tolerance and surface finish inspection to packaging and labelling. Tolerances and all surface finishes, for instance, are inspected with calibrated equipment. This helps ensure the highest quality possible.
Completely documented
All equipment and components in the Tri-Clover UltraPure portfolio are supplied with Alfa Laval Q-doc, a comprehensive documentation package that provides full transparency of the entire supply chain, from raw material to final equipment delivery. This smoothes purchasing and installation procedures as well as facilitates qualification, validation and change control procedures. Based on GDP (Good Documentation Practice), Alfa Laval Q-doc covers every aspect of UltraPure equipment supply and provides customers with transparent and well-documented quality assurance of the sourced equipment.
The Q-doc package for LKC UltraPure comprises quality and manufacturing procedures, material certificates and full traceability. This attention to detail maximizes uptime and minimizes risk.
For more information about standards and certificates, please visit our BioPharm Portal.
Product Benefits
- Safe and easy installation
- High and consistent quality
- Completely documented
Product catalogue
How it works
Application
Tri-Clover® LKC UltraPure is a non-return valve preventing reverse flow in a system. The UltraPure execution is designed and documented to meet the demand in industries like BioPharm and Personal Care.
Working principle
The spring acts on the valve plug and keep the valve closed until the force from the pressure in the inlet exceeds the force of the spring. If a reverse flow should occur the spring force and the pressure from the outlet will keep the valve closed.
Standard Design
The valve body is made in two parts that are assembled with a clamp ring. A guide disc and four legs guide the spring loaded valve plug in the valve body.
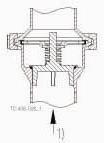
1 = Flow direction.
Shows the optimal built-in situation to make sure the valve is drainable. The four guide legs of the valve cone ensures good alignment.
Alfa Laval is proud to partner with John Brooks Industrial Automation
John Brooks Ltd signed an agreement with Alfa Laval in 2017 to represent and distribute Alfa Laval hygienic fluid handling equipment, such as butterfly valves, centrifugal pumps, tank cleaning heads and many flow control products from their range in New Zealand.

